Last week, we talked about some clues to water quality that you can see, like what lives in a stream. Today we’ll talk about an invisible part of water quality: pH.
The pH scale measures how acidic or basic water is:
- An example of an acid is lemon juice (acids often taste sour).
- An example of a base would be soapy water (bases often feel slippery).
- Plain old water from your sink should be neutral (in the middle between acidic and basic).
- Ever done the science experiment where you mix baking soda and vinegar , and it bubbles up like a volcano? That’s because you mixed a base and an acid!

Can you think of some items around the house that might be an acid or base? (Here are some examples to compare).
The pH scale ranges from 0-14. A pH 7 is neutral (neither an acid or a base). Anything lower than 7 (zero to 6.9) is an acid. Anything higher than 7 (7.1 to 14) is basic. (find more info on acids, bases and the pH scale here).
The more extreme numbers, like zero or 14, can burn and hurt you! But substances close to 7 are gentle and harmless.
Chemistry Moment
(optional!)
So what does pH actually measure? You might know that water is also called H2O in chemistry. That means water is made up of two hydrogen atoms (H2) and one oxygen atom (O).
H2O molecules have negative and positive ends (like a battery or magnet). Sometimes, this attracts H2O molecules to each other. But when the H2O molecules join together, they leave extra hydrogen ions laying around. Some of those hydrogen ions are positively charged, and some are negative.
Acidic water has more positive hydrogen ions in it. Basic water has more negatively charged ions in it.
We will focus on how pH impacts life in water, and won’t worry much about the chemistry.
Why does pH matter to fish and bugs?
Think about the examples of acids and bases. Do you think the aquatic critters we have learned about would want to live in lemon juice or soapy water?
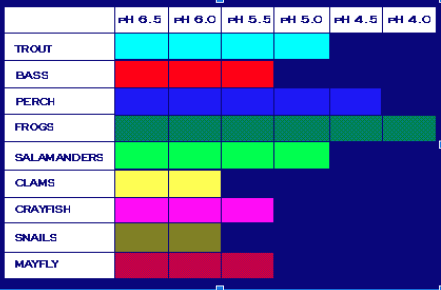
Aquatic life (things that live in water) is very sensitive. Most species do best in neutral water.

Remember the pollution tolerant and intolerant species that Kylee wrote about last week? Many fish and bugs are intolerant of extreme pHs.
If the water in a stream, lake, or puddle drops below a pH of 6, life will start to disappear. Similarly, if the pH rises above 8, our aquatic friends will find a new home.
Our watershed team at Rural Action tests the pH of nearby creeks often. If it is too low, that tells us something is wrong.
Test pH at home
Now for some fun! Let’s test some liquids at home. Are they acids, bases, or neutral?
You can do this by making an indicator solution out of cabbage and hot water. Indicator solutions change colors if a solution is an acid or a base.
What you will need:
- A small red cabbage
- Boiling water
- Strainer
- Small cups (clear cups or white pixie cups work best)
- Medicine dropper
- Household items to test
- You can test any liquid.
- Some suggestions are juices, tap water, and saliva.
- Household cleaning items, like soap and detergent, are interesting to test. But you MUST talk to an adult first. Some of them are dangerous to touch.
Note: If you can’t get these supplies, litmus paper can be ordered online or you can find pH test strips from a local pool or fish store. These can be used in place of the indicator solution to test your items. Another fun option is this Virtual PH Experiment – no supplies required!
Follow the directions for making and using the indicator solution here.
While your solution is cooling during step 3, gather you some safe liquids to test.
Before you start testing, hypothesize whether you think each of your items will be an acid or a base.
Record your test results. You can make an Acid/Base foldable like this one, and write your results on the correct side.
To go deeper:
Try testing the pH of a creek, pond, or puddle near you.
Is it close to neutral pH of 7? If not what do you think are some factors that might be causing this? Is there run-off from a nearby road or something else that might change water’s pH?
If you have litmus paper or pH test strips, try using these to test the accuracy of your cabbage solution.
Wrapping up:
- How did your hypotheses compare to the actual results?
- Was there anything that surprised you
- Did you test the accuracy of your indicator solution? How close was it to the litmus paper or pH strips?
- Share your results with us!